
Like many of my peers, the COVID-19 pandemic has shaped my graduate school experience. I joined the Department of Evolution, Ecology and Organismal Biology in 2018 as a master’s student in Agus Muñoz-Garcia’s lab, where we study ecophysiology with Diploptera punctata, the Pacific Beetle Cockroach. My thesis project was initially quite lab-intensive and required major funding sources, which had to be reallocated once the pandemic started. This required some creativity and shifting in my own thesis to still answer the research questions I was interested in while using data was already available to me.
For my thesis project, I used data collected from a 2018 experiment that examined the consequences of food quality on the plastic response of organ masses, food consumption and assimilation, and energy expenditure of cockroaches.
We assigned animals to four different diet groups across a 60-day period: The high-quality group ate only dog food; the low-quality group ate a mixture of dog food and insoluble fiber; and two groups had diet switches incorporated every 20 days, where one group started off with a high-quality diet and the other with a low-quality diet. In each group, diets were switched at day 20 and switched again to the original diet at day 40.
We hypothesized that phenotypic plasticity of organ mass and activity is the link between environmental conditions of food availability and resource allocation. We predicted that changes in food quality would yield changes in organ mass, which affects the energy expenditure of the organ and therefore the patterns of resources distribution in the whole organism. We predicted that phenotypic changes in the traits we measured would be completely reversible.
We found partial support for this hypothesis: Some things, such as the digestive tract and carcass mass, did change in size in response to a change in diet. However, when individuals were exposed to their original diets after the initial switch, it seems this plasticity was not reversible, and the digestive tract and carcass mass did not return to their original levels.

We can explain these results in part by our other findings: Phenotypic plasticity was completely reversible in food consumption, where individuals eating low-quality food ate twice as much as individuals eating high-quality food while assimilating the same quantity. It seems the increase in food intake was associated with an increase in the digestive tract mass for these individuals, but after switching back to high-quality food, this change was not completely reversible.
We propose the diet that is first encountered may have implications on plasticity of size and activity of the digestive tract, which could have downstream consequences in terms of fitness. If an organism is stuck maintaining a larger digestive tract, an energetically expensive organ, even when environmental pressures have subsided, resource allocation decisions to other physiological processes, like maintaining reproductive organs, may be impacted. We found support for this idea: the degree of plasticity of the size of the digestive tract was associated with plasticity of energy expenditure, and the plasticity of the mass of abdominal organs, suggesting such tradeoffs are present. However, the sample sizes were small, so more data is needed to make a solid conclusion.
The molecular mechanisms underpinning phenotypic plasticity seem to be key in understanding its reversibility in some traits but not others. In conjunction with the Ohio State Nanomedicine lab, we have developed a technique for manipulating a key family of proteins in regulating cellular metabolism in D. punctata, the sirtuins.
Sirtuins are protein deacetylases that are tissue-specific, and generally use NAD+ as a cofactor, which makes their activity sensitive to the nutritional status of the cell. For our initial trials, we loaded plasmids into Engineered Extracellular Vesicles (EEVs) derived from mammalian cells, which allow for integration of the plasmid into the host cells.
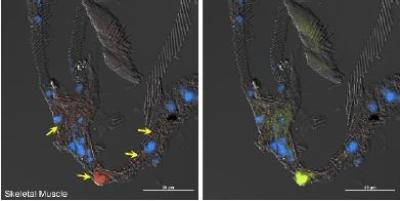
Preliminary experiments in which we injected EEVs loaded with genes for fluorescent protein showed that D. punctata cells successfully incorporated the plasmids and expressed the gene carried. The next step will be to load plasmids containing a subtype of sirtuin into the EEVs, to initiate overexpression in D. punctata and see if there is an effect on phenotypic plasticity of organ mass, energy expenditure, and food consumption and assimilation. This, of course, will be an endeavor for a future graduate student. But I am excited to see the results
Any well-rounded scientist should be able to not just do solid research but be able to have conversations about their research with audiences of various backgrounds. Working in EEOB has afforded me opportunities to grow in how I communicate science: from the college students enrolled in the recitations and labs I have led, to local girl scout troops checking out the cool arthropods from the insectary, to tipsy adults trying their unsteady hands at building circuits at COSI’s After Dark events.

I definitely have been bitten by the outreach bug and plan on continuing to volunteer now that I have graduated. To the new graduate students: I recommend trying everything you think is even a little interesting, because you never know what kind of connections you will make or the opportunities you will encounter. Volunteer your time in the Graduate Evolution & Ecology Student organization, take a seminar that is out of your wheelhouse but could be interesting, sign up to TA a course that is not directly related to your field but would provide you a well-rounded background. Even in the midst of a global pandemic, there are still connections to be made, collaborations to foster and new ways to challenge yourself.
